I saw the new Hospira pump - you know, the one with the funny name (okay, I'm writing this on the plane and can't remember the Symbiq). It has a very nice color touch screen that covers almost the entire front of the device. The device has an 802.11b radio presently, but will have an a/b/g radio in the third quarter. Surprisingly, there is no patient name displayed on the pump. The helpful and patient folks in the booth attributed this to HIPAA requirements; either their product manager's don't understand HIPAA, or this is spin for what will be an increasingly important question. This apparent short coming could be a consequence of Hospira's dependency on third party meds admin applications to establish patient context. (The good news is the display is all software, so adding the name should be relatively easy.) The back of the pump has a heavy duty faux metal mount for attaching the pump to a pole.
Baxter's booth was a bit slow, having recently come off their FDA recall and ship hold. They didn't have any new products. The wireless radio they showed at HIMSS 2 years ago in Dallas is still not available. Nor is the wireless PDA they showed that established patient context and provided nurse carried alarm notification.
Cardinal's Alaris pumps remain the only infusion pumps to establish patient context on the medical device (the safest most reliable way to associate data coming out of a medical device) - without any additional third party software (although I doubt it's any less expensive than Hospira's multi vendor solution).
Neither B Braun or Sigma were exhibiting at the show.
You may ask, "Why obsess over patient context?" Without patient context, "smart" pumps can only provide oversight of pump configuration based on the med being administered - there is no consideration of the patient (that might determin dose) or the patient's order (that could indicate the wrong medicine). Also, with out patient identification (and caregiver ID) any QA database is anonymous - a very blunt tool for improving safety. Finally, it's hard to export pump data into a medical record, provide surveillance, or do alarm notification beyond the pump itself without patient context. With the increasing push on patient safety patient identified data will become increasingly important.
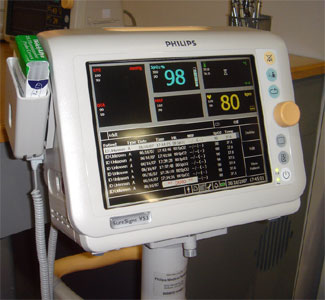
Philips had a nice customer appreciation event one night where I managed to pick up some information on their new SureSigns VS3 vital signs monitor and Philip's HL7 implementation. The VS3 reminds me of the saying, "If your only tool is a hammer, every problem looks like a nail." Being strong in high acuity patient monitoring, the VS3 looks more like a continuous monitor than a spot vital signs monitor. In fact, while many vital signs monitors only display numeric data, the VS3 can also show waveforms (SpO2) and trended data. Like the VM line, the VS3 uses a bar code reader to establish patient context. The system does not support an ADT interface, so you can't identify the patient from a pick list - important if you don't already use bar code patient IDs.
The device is not wireless - could this be the result of their commitment to WMTS for all their patient monitors? Some customers might balk at installing WMTS house-wide just for spot vital signs. Consequently, the VS3 (like all their VM low acuity monitors) has an RJ45 Ethernet port on the back of the device. This forces a use model where the caregiver gathers a queue of data as they take readings from patient after patient. When they're done, they must park the monitor where it's plugged in to recharge and connected to the network for a batch data download. To support this use model, the VS3 presents a nice listing readings taken in the bottom half of the big luxurious (for a vital signs monitor) color display. The downside of this approach is the potential for delays in the data getting on the chart, either because the caregiver was interrupted while taking readings, or they forgot to plug in the network cable when they were done. Wired network connections for portable devices also suffer from the not uncommon occurrence when forgotten cables jerk out the wall plate, frequently disabling the network port. Wireless connectivity avoids these limitations.
Philips was the first vendor to provide direct HL7 output from their medical devices. Market acceptance has been pretty good. The devices themselves require a server that aggregates feeds from multiple monitors, and the devices have some configurability. The longer term plan is to eliminate the server so monitors can communicate directly with the host system. It would be interesting to see how this would work out.
GE showed the new Carescape Data Module. This update of the Tram data module provides data continuity across multiple GE patient monitors. When originally conceived, most patient monitors were not portable and patient transport was done with special monitors. Nowadays, most monitors are portable and the use of transport monitors is limited. The module has cabling to support two use models. When placed near the patient, the Data Module uses shorter sensor cables to the patient, providing reduced chance of patients getting tangled up in lead wires. In this case, a single long cable connects the Data Module to the patient monitor. The other use model uses a shorter cable between monitor and Data Module and better supports traditional transport use cases. The Data Module has its own battery for transport, and can even power the portable monitor if that monitor's battery is not charged.
GE was also showing version 5 of the Carescape CIC Pro central station, and Carescape Mobile Viewer. The old Patient Viewer only supported the Unity Network (and the old Marquette patient monitors), the new version also displays data from Datex patient montors. The Mobile Viewer provides remote surveillance on PCs, tablets, PDAs and cell phones. While alarm conditions are shown, the product does not provide alarm notification.
GE also showed their Dynamap with an optional infrared (IrDA) communications port. The IrDA port allows the spot vital signs monitor to transmit data to a CareFusion PDA for subsequent uploading to the EMR. Since the acquisition of CareFusion by Cardinal Health, it is not clear what GE plans to do long term - nor is it clear that Cardinal has any interest in continuing CareFusion's OEM business rather than focusing exclusively on their own point of care (PoC) product strategies.
Also on the monitoring front, Welch Allyn noted a refresh on their MicroPaq - incorporating the latest Masimo SET board and their new 802.11a/b/g radio. The new device will weigh the same, but be a bit thicker at the top. Colin showed the BP-S510 that will be released next month. The Colin Prodigy II spot vital signs monitor incorporates new wired Ethernet connectivity. CAS was also showing their vital sign monitors. Mindray was showing a number of devices - conventional telemetry pack, low acuity monitor and a portable ultrasound system. Connectivity was pretty basic with a simple central station and RS232 for data export. Goldway, another Chinese device vendor, was showing a couple low acuity monitors. I've got to wonder how successful vendors will be with low cost manufacturing business models. While device prices are high (I mean how many ICU patients really need a $42,000 monitor?), market requirements have evolved beyond the box itself. Most vendors spend too much for connectivity features, but off-shore vendors also have the challenge of getting good requirements.
Pictured right is the Philips VS3 vital signs monitor.
UPDATE: In reviewing the bits on monitoring vendors above, I realized I forgot Nihon Kohden. They had a pretty big island booth and showed their ZS-940P patient worn low acuity patient monitor. This device received prominent position in their booth (photo).


Recent Comments