Okay, it's not all about workflow, just mostly, as you'll see.
A while back Ann Farrell was nice enough to bring an interesting paper to my attention. Titled, "Workarounds to Barcode Medication Administration Systems: Their Occurrences, Causes, and Threats to Patient Safety" the paper is a fascinating read for several reasons. The authors studied barcode medication administration systems (BCMA) at 5 hospitals, and identified 15 types of workarounds and 31 types of causes of workarounds. This paper provides the most detailed and comprehensive description of product and implementation shortcomings centered on the point of care that I've ever seen. It's devastating. Really.
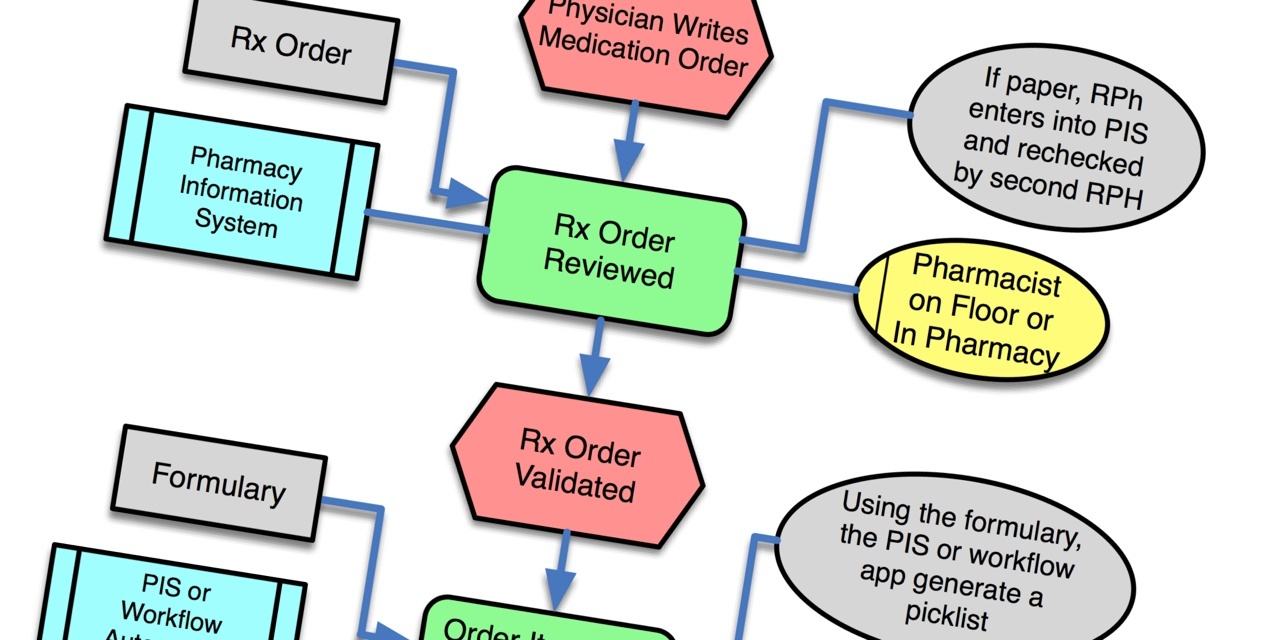
So what's this got to do with medical device connectivity? Two words: workflow and barcodes. Medical device connectivity is the automation of workflow through the integration of medical devices and information system. Likewise, BCMA is the automation of workflow through the use of auto ID (barcode labels and scanners) and information systems. With connectivity, attention centers on the connection; with BCMA, attention centers on the barcoding. Where should the attention be focused? Workflow.
Workflow is the Rodney Dangerfield of point of care systems. Everyone, manufacturers and clinicians both, focus on the tangible stuff, like serial cables and network connectivity or barcodes and readers. The invisible stuff, like water is to fish, is the workflow that occurs at the point of care. There are two key workflow data points that are required for a well designed product. First is capturing the complete workflow process in which the technology solution is to be used.
Framing the workflow should extend beyond the scope of the actual product, so that everything flows well at both the initiation and end of the workflow. Whether you're a provider looking to define requirements for a vendor selection process, or a manufacturer developing a new product, use cases are an ideal tool for capturing workflow. Use cases are easy for non-engineers (product managers, application specialists, clinical analysts) to understand and use and can be structured to provide engineers with something that can easily be translated into software specifications.
The other key factor for good product design is managing workflow variability. After you've done a few workflow exercises you'll see that most of any workflow is remarkably similar across users. But there's always some portion(s) of the workflow that are highly variable. These areas of variation must be identified. Then the challenge is to define the degree of variability for each of the variations you're willing to support in your product.
For every percentage of variability your product does not support, you can expect a certain percentage in lost sales or dissatisfied customers who make the mistake of buying a product that doesn't match their workflow. Thoroughly understanding workflow variability enables you to make an informed decisions about how much of your addressable market you want to trade off against the added R&D cost to support the target market's full variability. Once you've captured the variability in your target workflow, you can make informed trade off decisions -- and if you decide to support all or part of that variability you've likely captured what you need for requirements.
So let's go back the Koppel paper on BCMA. First, the methodology used to gather the data for their study was similar to what a would be required to capture workflow. They used a combination of structured observations where they directly observed clinicians going about their work over a variety of representative time frames (in representative units, over each shift, at shift change, etc.). This is typically the first phase in gathering use cases. Next, the authors held unstructured and semi-structured interviews with groups of participants. This is a good method to nailing workflow variability, building on a solid understanding of the basic workflow gathered by direct observation. Finally, a couple of the authors participated in staff meetings about BCMA use.
I am sometimes amazed at the amount of "voice of the customer" data a manufacturer can collect, without actually understanding and documenting the actual workflow. Providers too tend to miss the workflow opportunity -- on site clinical trials of new systems are a common vendor selection tool, but it is very rare when a provider actually documents their workflow and uses that to provide detailed requirements to prospective vendors.
These missed workflow opportunities, with vendors early in product development and providers at vendor selection, are why reading this paper just makes me cringe. The authors divided BCMA-related workarounds into 3 groups:
- Omission of process steps;
- Steps performed out of sequence; and
- Unauthorized BCMA process steps.
Probable causes of BCMA workarounds fell into 4 categories:
- Technology related;
- Task related;
- Organizational; and
- Patient related.
Reading the actual descriptions of workarounds and their probable causes points to the following shortcomings:
- Just plain old poor user interface design -- shame on both the vendor and the providers who bought systems with these problems.
- Poorly automated workflow -- either the vendor didn't understand the workflow, or made a decision to "just have the user do it this way" in spite of the recognized workflow, and again shame on providers who didn't recognize these shortcomings due to a lack of understanding their own workflows.
- Poor planning or implementation -- buying computers on wheels (COWs) that don't fit through doorways or actually reach the bedside (30' cords for barcode readers are not the answer) are the responsibility of the vendor to ensure the customer buys the right thing, and ultimately the responsibility of the buyer to, you know, buy the right thing.
Now we come to one of my pet peeves, barcoding. I don't really understand why the terms "auto ID" and "barcode" are used together. The set up for this requires the production and application of a barcode to a drug, device, patient, etc. And the user must manually pick up the reader and scan the barcode. This works great in the grocery store, but not in hospitals. If it wasn't for the fact that (when they can be read) barcodes eliminate the potential for typographical errors, barcodes would represent more work than simply using a keyboard. Barcode's saving grace is that barcode labels are pretty cheap, and in the best utilization of the technology are printed by the manufacturer as part of the product packaging. Again, think of the grocery store.
Barcodes are so simple conceptually, that many discount the planning and engineering required to design and implement a system that works reliably. Many of the workarounds noted in the Koppel paper result from the inability to read the barcode. Getting barcodes that can be reliably read on patient's wristbands is not a trivial task. You must assemble the right combination of barcode printers, ink, wrist band material, and barcode symbology and orientation (you can't read a linear barcode that wraps 2 inches around the patient's wrist -- you'll never get it flat enough to scan). Another big problem with BCMA is that many of the barcodes on the meds administered to patients have to be produced and applied by the hospital.
Earlier this week I was interviewed for a magazine article singing the praises of barcodes in general, and meds administration in general. I asked the writer what market adoption rates for BCMA they'd uncovered. Over the past two years adoption grew from 23% to 25%. Certainly we've know about the need since the publication of the IOM's publication of To Err is Human in 1999. The adoption of BCMA is not low and slow because hospitals don't care. Koppel's paper does a lot to shine a light on some of the reasons adoption has lagged. I'll be surprised if I get quoted in the barcode story.


Recent Comments