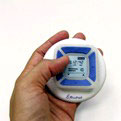
Synova Healthcare announced last week that a Synova subsidiary and development partner BioPad Ltd. successfully produced the initial set of antepartum fetal-movement
monitors for use in their first "in-home" clinical trial. The device is
designed to provide a non-invasive means of monitoring fetal movement
during the last trimester of pregnancy (press release).
trimester (and often earlier), and can be performed at-home manually by
the mother herself. Manual fetal monitoring is currently done while the
mother lies quietly on her side and counts the number of fetal
movements within a given time frame, or the time necessary to detect a
given number of fetal movements. The non-invasive fetal movement
monitor currently being co-developed by Synova and BioPad is aimed at
improving this practice by increasing the accuracy agreement of the
measurement via this non-invasive device versus manual measurement by
the expectant mother, when compared with ultrasound. The Company
believes this will reduce maternal anxiety (as the fetal monitor is
expected to identify fetal movement the mother may miss) and may
contribute to improved outcomes by assisting in identifying real
emergencies when they occur and potentially providing the time
necessary to intervene successfully.
Intended as an over the counter (OTC) product, the unit includes the sensor, signal processing and a small alpha numeric display. Pictured right is a photo of the sensor.
[Hat tip: Wireless Healthcare]


Recent Comments